Chris Nelson

Edwards Lifesciences identified the top data releases from 2022 that contributed most to shaping awareness about the extent and impact of underdiagnosis and undertreatment of aortic stenosis (AS).

New American Heart Association scientific statement outlines research on complementary and alternative therapies for heart failure.
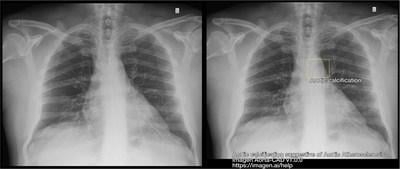
Imagen Technologies, Inc. announced the U.S. Food and Drug Administration’s 510(k) clearance of the computer-assisted detection (CADe) device Aorta-CAD. This new, FDA cleared device is designed to assist physicians at detecting findings on chest X-rays that are suggestive of Aortic Atherosclerosis and Aortic Ectasia.

Heather Caron, RCES, RCIS, provides a brief review of best practices for EP lab efficiency at Gundersen Health System.

Vascular Disease Management speaks with Interventional Radiologist Nathan Fagan, MD, from Nationwide Children’s Hospital in Columbus, Ohio, about using the ARTIS icono in the interventional pediatric space.

The American Heart Association, a global force for longer, healthier lives for all, is supporting social and tech entrepreneurs who are driving change by developing new and innovative solutions to address the social determinants of health in communities across the country.

The R2P™ DESTINATION SLENDER™ Guiding Sheath is a dedicated radial sheath developed by Terumo. We present a series of challenging radial cases with the use of the R2P™ DESTINATION SLENDER™ Guiding Sheath.
Danvers, Mass., October 31, 2022 – Abiomed (Nasdaq: ABMD) announces that Impella RP Flex with SmartAssist has received U.S. Food and Drug Administration (FDA) pre-market approval (PMA), the FDA’s highest level of approval, as safe and effective to treat acute right heart failure for up to 14 days.

Fewer than half of veterans, most of whom were not on lipid-lowering therapy before an event, received intensified lipid-lowering therapies after an MI or elective revascularization, researchers reported.

GENEVA, Oct. 18, 2022 /PRNewswire/ — Swiss-based medical technology company MedAlliance has announced it has entered into an agreement with Cordis for an acquisition which includes an initial investment of $35M and upfront closing payment of $200M, regulatory achievement milestones of up to $125M and commercial milestones of up to $775M through 2029 for a total consideration of up to $1.135 Billion.
Stay Up-To-Date on jobs and industry news.
Sign up for the CathLab.com newsletter today!
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact

