DAIC Articles
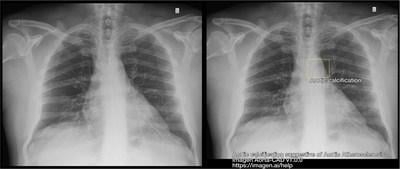
Imagen Technologies, Inc. announced the U.S. Food and Drug Administration’s 510(k) clearance of the computer-assisted detection (CADe) device Aorta-CAD. This new, FDA cleared device is designed to assist physicians at detecting findings on chest X-rays that are suggestive of Aortic Atherosclerosis and Aortic Ectasia.
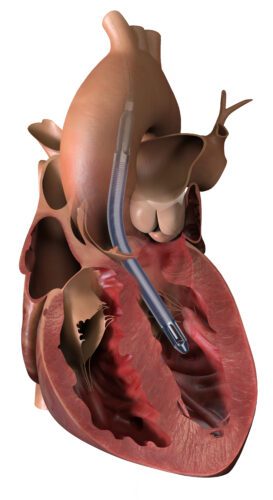
Hackensack Meridian Health, Hackensack University Medical Center cardiac surgeon Yuriy Dudiy, M.D. became the second in the world to successfully implant the Impella Bridge-to-Recovery (BTR) heart pump for the technology’s U.S. Food and Drug Administration (FDA) early feasibility study (EFS) investigational device exemption (IDE). Hackensack University Medical Center is one of only five hospitals in the U.S. selected […]
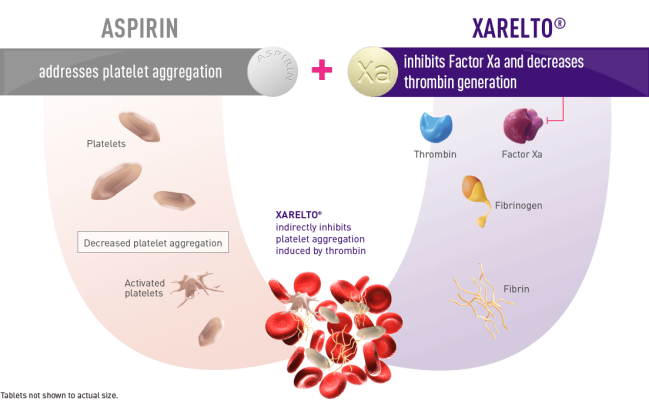
COMPASS open label extension study results support the long-term use of XARELTO plus aspirin for vascular protection in patients with chronic CAD and/or PAD

Physician-scientists in the Smidt Heart Institute at Cedars-Sinai have created an artificial intelligence (AI) tool that can effectively identify and distinguish between two life-threatening heart conditions that are often easy to miss: hypertrophic cardiomyopathy and cardiac amyloidosis. The new findings were published in JAMA Cardiology.
The U.S. Food and Drug Administration (FDA) has cleared Novartis’ inclisiran (Leqvio), the first small interfering RNA (siRNA) therapy to lower low-density lipoprotein cholesterol (LDL-C) with two doses a year, after an initial dose and one at three months.

Boston Scientific Corp. has commenced enrollment in the HI-PEITHO clinical trial, a collaborative research study with the Pulmonary Embolism Response Team (PERT) Consortium and the University Medical Center of the Johannes Gutenberg University of Mainz comparing use of the EkoSonic Endovascular System in combination with anticoagulation to anticoagulation alone for the treatment of acute, intermediate-high-risk […]
Stay Up-To-Date on jobs and industry news.
Sign up for the CathLab.com newsletter today!
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact

