Chris Nelson
The United States Food and Drug Administration (FDA) has granted 510(k) clearance to Abiomed (Nasdaq: ABMD) for its Impella Low Profile Sheath. Compared to the existing 14 French (Fr) sheath used for placement of Impella CP, the new sheath maintains the same inner diameter, but reduces the outer diameter by nearly 2 Fr.

In patients with ascending thoracic aortic aneurysm (TAA) and no other organ-system abnormalities, the absolute risk of aortic dissection is low but increases with larger aortic sizes, according to new population data.https://www.tctmd.com/news/aortic-dissection-risk-rises-thoracic-aneurysm-size-large-network-study.

In this article, the team from University of North Carolina (UNC) Health Rex discuss the unique shared staffing model for their cardiovascular procedures, including for LAAO cases.
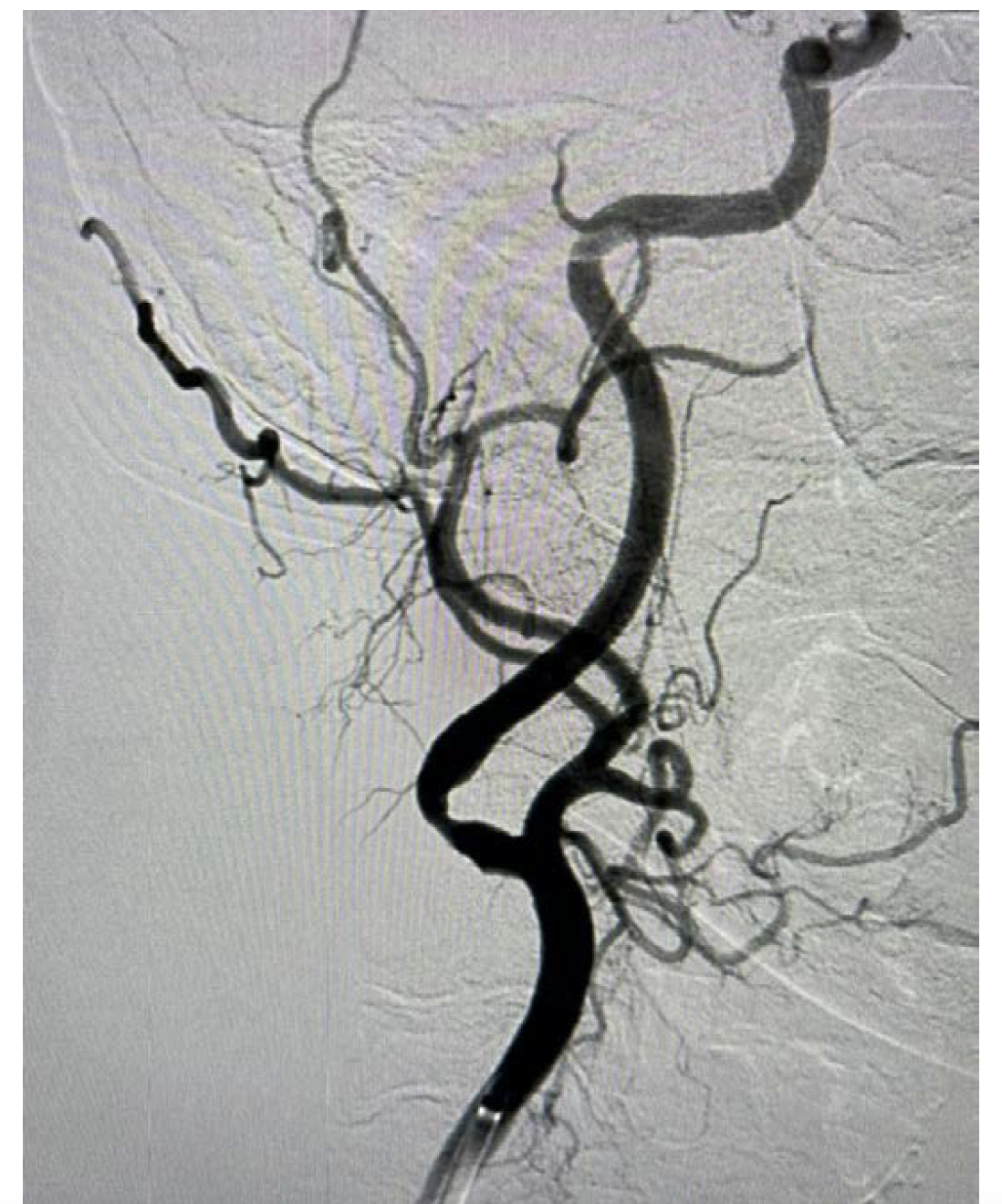
Case Study: This case illustrates the potential for complications when facing severely fibrotic lesions in carotid catheterization as well as potential options for future operators.

Surgery is the mainstay of treatment for patients with acute aortic regurgitation (AR). Bioprosthetic valves (BVs) demonstrate an excellent profile in terms of hemodynamics and seem to be a good fit for patients with high risk of bleeding. Nonetheless, the durability of BVs is limited compared to mechanical valves; inevitably, BV degeneration may lead to […]
BOSTON–(BUSINESS WIRE)–Results of a new per-protocol analysis of the ST-segment Elevation Myocardial Infarction Door-To-Unload (STEMI DTU) pilot trial data show significantly reduced infarct size in patients who received left ventricular (LV) unloading with Impella CP for 30 minutes prior to their percutaneous coronary intervention (PCI) compared to patients who received LV unloading followed by immediate PCI.
DANVERS, Mass. – September 16, 2022 – Abiomed (Nasdaq: ABMD) announces two approvals from the U.S. Food and Drug Administration (FDA) related to clinical research of Impella® heart pumps in acute myocardial infarction (AMI) cardiogenic shock patients.
IRVINE, Calif., Sept. 15, 2022 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), today announced the company’s PASCAL Precision transcatheter valve repair system for transcatheter edge-to-edge repair (TEER) has received FDA approval for the treatment of patients with degenerative mitral regurgitation (DMR).

Artificial sweeteners used as sugar substitutes in food and drinks may be associated with increased risk of CVD events and strokes, suggest data from the large NutriNet-Santé study of French adults.
IRVINE, Calif., Sept. 12, 2022 /PRNewswire/ — Edwards Lifesciences (NYSE: EW) today announced the launch of the SAPIEN 3 Ultra RESILIA valve, which incorporates Edwards’ breakthrough RESILIA tissue technology with the industry-leading SAPIEN 3 Ultra transcatheter aortic heart valve. The launch follows recent U.S. Food and Drug Administration (FDA) approval.
Stay Up-To-Date on jobs and industry news.
Sign up for the CathLab.com newsletter today!

