Cardiac Rhythm News

People who develop an arrhythmia after surgery have an increased risk of subsequently being admitted to hospital with heart failure, according to a study of over three million patients published in the European Heart Journal.

Use of an artificial intelligence (AI)-based app using electrocardiogram (ECG) signals recorded with an Apple Watch is able to identify left-ventricular dysfunction, research presented at the Heart Rhythm Society’s 2022 annual meeting (HRS 2022, 29 April–1 May, San Francisco, USA) has found.

Portable electronic devices containing magnets may inhibit pulse generators for implanted cardiac devices (ICDs) and pacemakers according to new research published in Circulation: Arrhythmia and Electrophysiology.
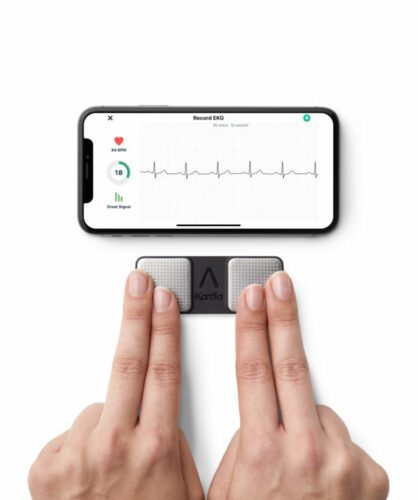
The National Institute for Health and Care Excellence (NICE) has issued Medical Technologies Guidance (MTG) recommending KardiaMobile (AliveCor) as an option for detecting atrial fibrillation (AF) in patients with suspected paroxysmal AF, who present with symptoms such as palpitations and are referred for ambulatory ECG monitoring by a clinician.

Abbott has announced that the US Food and Drug Administration (FDA) has approved the company’s Amplatzer Talisman PFO Occlusion System to treat people with a patent foramen ovale (PFO)—a small opening between the upper chambers of the heart—who are at risk of recurrent ischaemic stroke.

Economic analyses from the WRAP-IT study, sponsored by Medtronic, demonstrate the Tyrx cardiac absorbable antibacterial envelope’s (Tyrx Envelope) cost effectiveness in European markets for patients at increased risk of infections.
Stay Up-To-Date on jobs and industry news.
Sign up for the CathLab.com newsletter today!
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact

